As an unusually large and early seasonal surge of RSV cases inundate children's hospitals around the country, pharmaceutical giant Pfizer offered a glimmer of hope Tuesday in the form of top-line, phase three clinical trial results.
The company's experimental RSV vaccine—given to pregnant trial participants—was 82 percent effective at preventing severe RSV-related lower respiratory tract illness in the first three months of an infant's life. It was 69 percent effective over the first six months, Pfizer announced.
“We are thrilled by these data as this is the first-ever investigational vaccine shown to help protect newborns against severe RSV-related respiratory illness immediately at birth,” Pfizer Chief Scientific Officer Annaliesa Anderson said in a statement.
The company said it planned to file for regulatory approval from the Food and Drug Administration by the end of the year, which may mean a vaccine could be available in time for next year's RSV season.
The announcement is promising, but there are reasons for caution, too. The company has only released top-line results in a press release, for one thing. The data will have to go through more detailed outside review. Pfizer also noted that the vaccine failed to meet the second of the trial's two primary goals, which was to reach the pre-determined statistical criteria for efficacy against non-severe RSV-related lower respiratory tract illness—though the company says some efficacy was clinically meaningful.
Still, there is reason to be excited by Tuesday's news, which follows decades of struggle by researchers trying to fight RSV. That includes a disastrous vaccine trial in the 1960s, which caused vaccinated children to develop more severe disease from an RSV infection and led to the tragic death of two infants.
An oft-overlooked virus
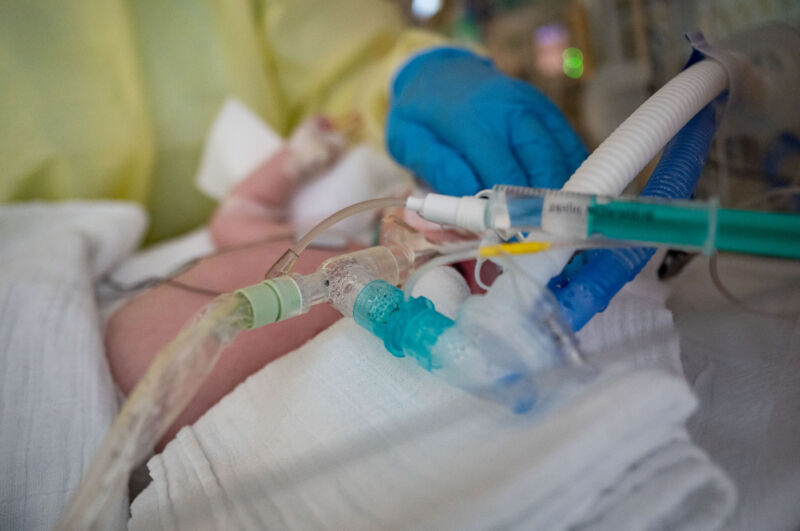
It may seem newly famous, but RSV—or respiratory syncytial (sin-SISH-uhl) virus—is a common, seasonal virus that has long posed a grave risk to infants and toddlers. Nearly everyone is infected during childhood, and most experience only a mild respiratory illness. But for a small fraction of children, particularly those under 5, it can turn life-threatening. RSV sends around 3.6 million to the hospital each year worldwide and kills over 100,000 children under 5 each year. Deaths most often occur in infants under 6 months old and among children in lower-income countries.
In the US, RSV is among the leading causes of hospitalization for children under 5. A typical RSV season sends between 58,000 and 80,0000 children under 5 to the hospital and kills between 100 to 300, the Centers for Disease Control and Prevention estimates.
Researchers have been working for decades to prevent and treat RSV. But a dark cloud loomed over the field for years, halting progress. It formed in the 1960s, when researchers began working on a vaccine against RSV. The experimental vaccine's design used a standard treatment of the times—heat and a solution of formaldehyde (formalin) to inactivate the virus and "fix" or stabilize its proteins. Thus, the formalin-inactivated virus vaccine could present a whole virion to the immune system that was incapable of infecting cells, yet had all of its antigenic components essentially frozen in place so immune cells could learn to target key components.
Catastrophic candidate
But the vaccine was a tragic disaster. Not only did it fail to protect children from RSV in several clinical trials in 1966, but it also appeared to make the children more vulnerable to severe RSV.
For example, in one small US trial, researchers gave infants between the ages of 2 months and 7 months a three-dose regimen. Of 40 unvaccinated infants in a control group, 21 caught RSV during a subsequent wave of infection in the community, and only one of the unvaccinated children required hospitalization. Meanwhile, of 30 infants given the experimental vaccine, 20 went on to catch RSV, but 16 (80 percent) required hospitalization. Two of the children later died of bacterial pneumonia that developed after their RSV infections.
In the decades since, researchers worked out how exactly the vaccine caused "enhanced respiratory disease" (ERD) syndrome in the vaccinated children. First, the formalin-inactivated RSV vaccine spurred weak antibodies that only feebly blocked and neutralized live virus. This impotent response led to an accumulation of antibody-virus immune complexes that, in turn, activate exacerbating immune responses, including inflammation. The vaccine also spurred T cell responses that can cause exaggerated inflammation in the lung upon subsequent RSV infection. All of this can pave the way for severe disease and complications, such as bacterial pneumonia.


3175x175(CURRENT).thumb.jpg.b05acc060982b36f5891ba728e6d953c.jpg)

Recommended Comments
There are no comments to display.
Join the conversation
You can post now and register later. If you have an account, sign in now to post with your account.
Note: Your post will require moderator approval before it will be visible.