Getting oxygen from regolith takes 24 kWh per kilogram, and we'd need tonnes.
If humanity is ever to spread out into the Solar System, we're going to need to find a way to put fuel into rockets somewhere other than the cozy confines of a launchpad on Earth. One option for that is in low-Earth orbit, which has the advantage of being located very close to said launch pads. But it has the considerable disadvantage of requiring a lot of energy to escape Earth's gravity—it takes a lot of fuel to put substantially less fuel into orbit.
One alternative is to produce fuel on the Moon. We know there is hydrogen and oxygen present, and the Moon's gravity is far easier to overcome, meaning more of what we produce there can be used to send things deeper into the Solar System. But there is a tradeoff: any fuel production infrastructure will likely need to be built on Earth and sent to the Moon.
How much infrastructure is that going to involve? A study released today by PNAS evaluates the energy costs of producing oxygen on the Moon, and finds that they're substantial: about 24 kWh per kilogram. This doesn't sound bad until you start considering how many kilograms we're going to eventually need.
Free the oxygen!
The math that makes refueling from the Moon appealing is pretty simple. "As a rule of thumb," write the authors of the new study on the topic, "rockets launched from Earth destined for [Earth-Moon Lagrange Point 1] must burn ~25 kg of propellant to transport one kg of payload, whereas rockets launched from the Moon to [Earth-Moon Lagrange Point 1] would burn only ~four kg of propellant to transport one kg of payload." Departing from the Earth-Moon Lagrange Point for locations deeper into the Solar System also requires less energy than leaving low-Earth orbit, meaning the fuel we get there is ultimately more useful, at least from an exploration perspective.
But, of course, you need to make the fuel there in the first place. The obvious choice for that is water, which can be split to produce hydrogen and oxygen. We know there is water on the Moon, but we don't yet know how much, and whether it's concentrated into large deposits. Given that uncertainty, people have also looked at other materials that we know are present in abundance on the Moon's surface.
And there's probably nothing more abundant on that surface than regolith, the dust left over from constant tiny impacts that have, over time, eroded lunar rocks. The regolith is composed of a variety of minerals, many of which contain oxygen, typically the heavier component of rocket fuel. And a variety of people have figured out the chemistry involved in separating oxygen from these minerals on the scale needed for rocket fuel production.
But knowing the chemistry is different from knowing what sort of infrastructure is needed to get that chemistry done at a meaningful scale. To get a sense of this, the researchers decided to focus on isolating oxygen from a mineral called ilmenite, or FeTiO3. It's not the easiest way to get oxygen—iron oxides win out there—but it's well understood. Someone actually patented oxygen production from ilmenite back in the 1970s, and two hardware prototypes have been developed, one of which may be sent to the Moon on a future NASA mission.
The researchers propose a system that would harvest regolith, partly purify the ilmenite, then combine it with hydrogen at high temperatures, which would strip the oxygen out as water, leaving behind purified iron and titanium (both of which may be useful to have). The resulting water would then be split to feed the hydrogen back into the system, while the oxygen can be sent off for use in rockets.
(This wouldn't solve the issue of what that oxygen will ultimately oxidize to power a rocket. But oxygen is typically the heavier component of rocket fuel combinations—typically about 80 percent of the mass—and so the bigger challenge to get to a fuel depot.)
Obviously, this process will require a lot of infrastructure, like harvesters, separators, high-temperature reaction chambers, and more. But the researchers focus on a single element: how much power will it suck down?
More power!
To get their numbers, the researchers made a few simplifying assumptions. These include assuming that it's possible to purify ilmenite from raw regolith and that it will be present in particles small enough that about half the material present will participate in chemical reactions. They ignored both the potential to get even more oxygen from the iron and titanium oxides present, as well as the potential for contamination from problematic materials like hydrogen sulfide or hydrochloric acid.
The team found that almost all of the energy is consumed at three steps in the process: the high-temperature hydrogen reaction that produces water (55 percent), splitting the water afterwards (38 percent), and converting the resulting oxygen to its liquid form (five percent). The typical total usage, depending on factors like the concentration of ilmenite in the regolith, worked out to be about 24 kW-hr for each kilogram of liquid oxygen.
Obviously, the numbers are sensitive to how efficiently you can do things like heat the reaction mix. (It might be possible to do this heating with concentrated solar, avoiding the use of electricity for this entirely, but the authors didn't analyze that.) But it was also sensitive to less obvious efficiencies. For example, a better separation of the ilmenite from the rest of the regolith means you're using less energy to heat contaminants. So, while the energetic cost of that separation is small, it pays off to do it effectively.
Based on orbital observations, the researchers map out the areas where ilmenite is present at high enough concentrations for this approach to make sense. These include some of the mares on the near side of the Moon, so they're easy to get to.
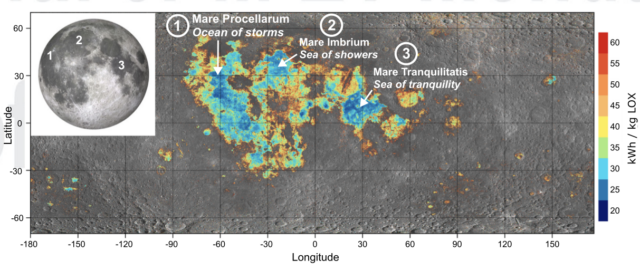
On its own, 24 kWh doesn't seem like a lot of power. The problem is that we will need a lot of kilograms. The researchers estimate that getting an empty SpaceX Starship from the lunar surface to the Earth-Moon Lagrange Point takes 80 tonnes of liquid oxygen. And a fully fueled starship can hold over 500 tonnes of liquid oxygen.
We can compare that to something like the solar array on the International Space Station, which has a capacity of about 100 kW. That means it could power the production of about four kilograms of oxygen an hour. At that rate, it'll take a bit over 10 days to produce a tonne, and a bit more than two years to get enough oxygen to get an empty Starship to the Lagrange Point—assuming 24-7 production. Being on the near side, they will only produce for half the time, given the lunar day.
Obviously, we can build larger arrays than that, but it boosts the amount of material that needs to be sent to the Moon from Earth. It may potentially make more sense to use nuclear power. While that would likely involve more infrastructure than solar arrays, it would allow the facilities to run around the clock, thus getting more production from everything else we've shipped from Earth.
This paper isn't meant to be the final word on the possibilities for lunar-based refueling; it's simply an early attempt to put hard numbers on what ultimately might be the best way to explore our Solar System. Still, it provides some perspective on just how much effort we'll need to make before that sort of exploration becomes possible.
PNAS, 2025. DOI: 10.1073/pnas.2306146122 (About DOIs).
Hope you enjoyed this news post.
Thank you for appreciating my time and effort posting news every day for many years.
News posts... 2023: 5,800+ | 2024: 5,700+ | 2025 (till end of January): 487
RIP Matrix | Farewell my friend ![]()



3175x175(CURRENT).thumb.jpg.b05acc060982b36f5891ba728e6d953c.jpg)


Recommended Comments
There are no comments to display.
Join the conversation
You can post now and register later. If you have an account, sign in now to post with your account.
Note: Your post will require moderator approval before it will be visible.