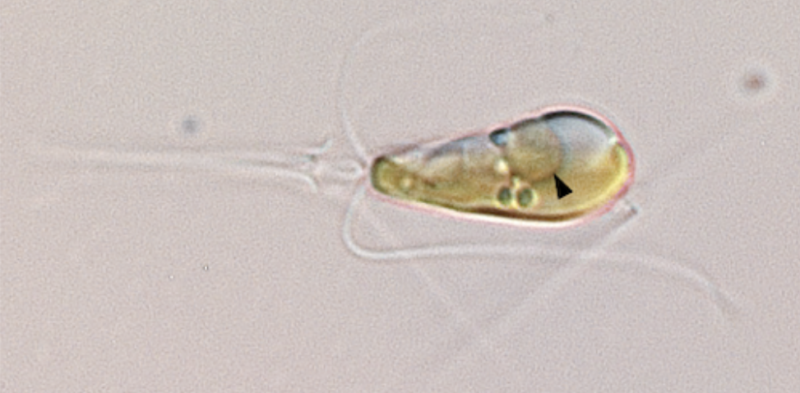
A photo of Braarudosphaera bigelowii with the nitroplast indicated by an arrowhead.
Tyler Coale
The complex cells that underlie animals and plants have a large collection of what are called organelles—compartments surrounded by membranes that perform specialized functions. Two of these were formed through a process called endosymbiosis, in which a once free-living organism is incorporated into a cell. These are the mitochondrion, where a former bacteria now handles the task of converting chemical energy into useful forms, and the chloroplast, where photosynthesis happens.
The fact that there are only a few cases of organelles that evolved through endosymbiosis suggests that it's an extremely rare event. Yet researchers may have found a new case, in which an organelle devoted to fixing nitrogen from the atmosphere is in the process of evolving. The resulting organelle, termed a nitroplast, is still in the process of specialization.
Getting nitrogen
Nitrogen is one of the elements central to life. Every DNA base, every amino acid in a protein contains at least one, and often several, nitrogen atoms. But nitrogen is remarkably difficult for life to get ahold of. N2 molecules might be extremely abundant in our atmosphere, but they're extremely difficult to break apart. The enzymes that can, called nitrogenases, are only found in bacteria, and they don't work in the presence of oxygen. Other organisms have to get nitrogen from their environment, which is one of the reasons we use so much energy to supply nitrogen fertilizers to many crops.
Some plants (notably legumes), however, can obtain nitrogen via a symbiotic relationship with bacteria. These plants form specialized nodules that provide a habitat for the nitrogen-producing bacteria. This relationship is a form of endosymbiosis, where microbes take up residence inside an organism's body or cells, with each organism typically providing chemicals that the other needs.
In more extreme cases, endosymbiosis can become obligatory. with neither organism able to survive without the other. In many insects, endosymbionts are passed on to offspring during the production of eggs, and the microbes themselves often lack key genes that would allow them to live independently.
But even states like this fall short of the situation found in mitochondria and chloroplasts. These organelles are thoroughly integrated into the cell, being duplicated and distributed when cells divide. They also have minimal genomes, with most of their proteins made by the cell and imported into the organelles. This level of integration is the product of over a billion years of evolution since the endosymbiotic relationship first started.
It's also apparently a difficult process, based on its apparent rarity. Beyond mitochondria and chloroplasts, there's only one confirmed example of a more recent endosymbiosis between eukaryotes and a bacterial species. (There are a number of cases where eukaryotic algae have been incorporated by other eukaryotes. Because these cells have compatible genetics, this occurs with a higher frequency.)
That's why finding another example is such an exciting prospect.
That’s no endosymbiont
The algae Braarudosphaera bigelowii seemed like it might be an interesting case. It clearly has an endosymbiotic cyanobacteria living in its cells, and there were indications that the bacteria had a compact genome, suggesting it had lost some genes. But, since we couldn't culture B. bigelowii, it was tough to assess the degree to which the bacteria had integrated with its host. But a large international team has now managed to get it to grow in the lab, allowing a more detailed characterization.
They found that a single internalized bacteria occupies a specific area within the cell's structure, near its posterior. Using isotopes as tracers, they found that carbon dioxide taken up by B. bigelowii was transferred to the bacteria. Meanwhile, the cell could also fix nitrogen. Since some cyanobacteria species are able to fix nitrogen, this was almost certainly due to the bacterial symbiote. Nitrogen-fixing only occurred during the day, suggesting the activity was integrated into the cell's metabolism.
A further sign of the bacteria's integration came when the researchers examined cell division. The bacteria is duplicated at the same time as the cell's mitochondria, and one copy is deposited in each of the two daughter cells.
The researchers separated out the two cells and purified proteins from each. They found that hundreds of proteins made by the algal cells ended up inside the bacteria, with their levels varying over the day/night cycle (more were present during daylight hours). Checking the genes that encode these proteins, the researchers found that they all shared a common sequence that directs them to the bacteria and then is clipped off when the protein is transported inside. Similar systems are used by mitochondria and chloroplasts.
The list of proteins found inside the bacteria showed that it contains everything necessary to fix nitrogen. At the same time, it doesn't have what's needed to use CO2 from the atmosphere as a carbon source, which is why it has to obtain its carbon from the surrounding cell.
An organelle, not a symbiote
All of these properties—the coordinated replication, the biochemical specialization, the existence of a system for importing proteins—are features of organelles, not endosymbiosis. So, the researchers conclude that what was once an endosymbiont has evolved into an organelle specialized in fixing nitrogen, and term it a nitroplast. This is only the fourth example of the evolution of an organelle, making it an impressive discovery.
Unlike things like mitochondria and chloroplasts, however, the nitroplast is limited to a single lineage of algae. That's likely because of its relatively recent origin; it's estimated that the relationship between the nitroplast and its host cell only dates aback about 100 million years, versus the billions of years for the other organelles. Still, its evolution would be extremely valuable for something like algae, which may live in environments where nitrogen sources are rare.
Might that change over time? The evolution of multicellularity is also fairly rare, and B. bigelowii would find itself competing with a huge range of existing multicellular species, so that seems unlikely. But a number of predatory cells have become photosynthetic by ingesting former free-living algae, and there's a chance that the nitroplast could spread this way, eventually enabling a range of single-celled algae to fix nitrogen.
So, this is unlikely to have a major impact on life on Earth anytime soon, barring a science-fiction future in which we understand the system well enough to engineer plant cells to host nitroplasts.
Science, 2024. DOI: 10.1126/science.adk1075 (About DOIs).



3175x175(CURRENT).thumb.jpg.b05acc060982b36f5891ba728e6d953c.jpg)

Recommended Comments
There are no comments to display.
Join the conversation
You can post now and register later. If you have an account, sign in now to post with your account.
Note: Your post will require moderator approval before it will be visible.