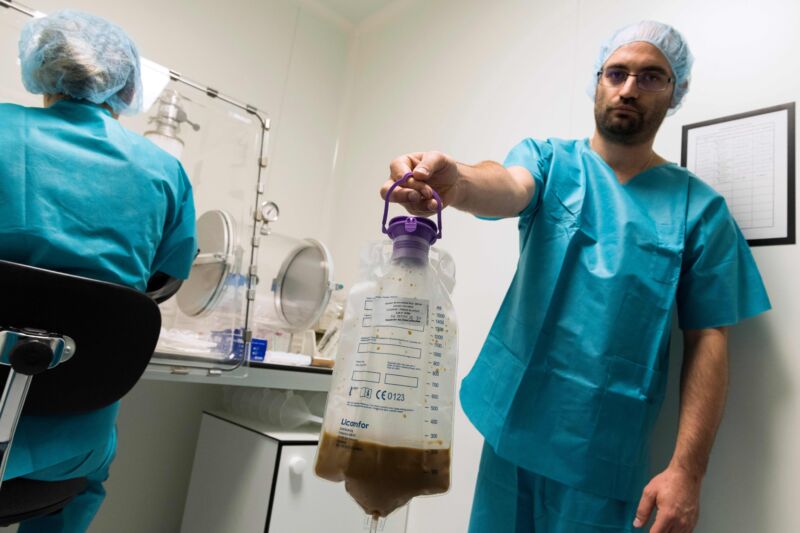
Laboratory technicians in France prepare stool to treat patients with serious colon infections by fecal microbiota transplantation (FMT), also known as gut flora transplant (GFT) in 2019.
For the first time, the US Food and Drug Administration has granted approval for a feces-based microbial treatment, which is used to prevent a recurring diarrheal infection that can become life-threatening.
The approval, announced Wednesday, is years in the making. Researchers have strained to harness the protective qualities of the complex, diverse, yet variable microbial communities found in healthy people's intestines and stool. Early on, rich fecal matter proved useful for restoring balance and blocking infection in those whose microbiomes have been disturbed—a state called dysbiosis, which can occur from disease and/or use of antibiotic drugs. But, our understanding of what makes a microbiome healthy, functional, and protective remains incomplete.
Doctors, meanwhile, pushed ahead, informally trying an array of methods to transplant fecal microbiota from healthy donors to the guts of patients—via enemas, tubes through the nose, and oral poop-packed capsules. Fecal microbiota transplants (FMTs) have been used to treat various ailments, from obesity to irritable bowel syndrome, to mixed success. But it quickly became apparent that FMTs were most readily effective at preventing recurrent infection from Clostridioides difficile (C. difficile or just C. diff).
C. diff bacteria cause diarrhea and significant inflammation in the colon. Severe infections can be life-threatening. In people with dysbiosis, C. diff can proliferate in the intestines, producing toxins that can lead to organ failure. Older people, those who are hospitalized, and people with weakened immune systems are particularly susceptible to C. diff, which can recur over and over in some vulnerable patients. In the US, C. diff infections are associated with up to 30,000 deaths per year.
With the pressing need for effective treatments against C. diff, regulators were forced to wade through the mucky issue of regulating and standardizing something as unruly and myriad as fecal matter. It also led to years of microbial sleuthing, synthetic slurries, stool donations, and clinical trials.
Solid success
Now, a product has finally floated to the top: Rebyota, a blend of donor stool, saline, and laxative solution given in a single treatment as an enema. It's teeming with heavily screened intestinal microbes at a concentration of 10,000,000 live organisms per milliliter. Its owner, Switzerland-based Ferring Pharmaceuticals, screens donors and their donated stool for a long list of infectious pathogens and other health factors.
In a Phase III clinical trial involving 262 participants—the results of which were published last month—Ferring's scientists reported that treatment with Rebyota led to a higher prevention rate of recurrent C. diff infections than in a placebo group at a rate of 70.6 percent in the treatment group compared with 57.5 percent in the placebo group. Prevention of C. diff was defined as an absence of C. diff diarrhea for eight weeks following treatment or placebo. The treatment was well tolerated, with no serious side effects. The FDA noted that given the variability of fecal matter, there is a potential that it could contain an unforeseen infectious agent or food allergens.
The approval of Rebyota is "an advance in caring for patients who have recurrent C. difficile infection," Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in an announcement. "Recurrent CDI impacts an individual’s quality of life and can also potentially be life-threatening. As the first FDA-approved fecal microbiota product, today’s action represents an important milestone, as it provides an additional approved option to prevent recurrent CDI."
Ferring—which acquired Rebyota in 2018 when it purchased its developer, Minnnesota-based Rebiotix—also celebrated the approval.
“We believe this is a major breakthrough in harnessing the power of the human microbiome to address significant unmet medical needs. This is the first FDA approval of a live biotherapeutic and the culmination of decades of research and clinical development," Ferring President Per Falk said. "Today’s announcement is not just a milestone for people living with recurrent C. difficile infection, but also represents a significant step which holds promise that many other diseases might be better understood, diagnosed, prevented, and treated using our rapidly evolving insights on the role of the microbiome in human health and disease."
- Karlston and aum
-

 2
2



Recommended Comments
There are no comments to display.
Join the conversation
You can post now and register later. If you have an account, sign in now to post with your account.
Note: Your post will require moderator approval before it will be visible.