Platinum is a ferociously expensive metal that is difficult to obtain and purify. Most of the small supply we produce every year isn't put to use for its properties as a metal. Instead, it's used as a catalyst for producing a variety of chemicals—and for cleaning up your car's exhaust. Everything made with platinum carries an added burden of cost and environmental damage because of that use.
This year, the Nobel Prize in Chemistry honors two researchers for helping to trigger a burst of research into catalysts that leave the metals behind. Benjamin List and David MacMillan made key discoveries that started the field of organocatalysis, developing catalysts that could be made from cheap, common chemicals. Their work took a disorganized set of anecdotes and gave them a strong conceptual footing that allowed many other labs to go further.
Not so metal
At their heart, chemical reactions involve the transfer of electrons, either between atoms or into new configurations of chemical bonds. Metals are often effective catalysts because they ease the process of transferring electrons. Many metals will easily make a temporary loan of their electrons during a reaction or, if properly prepared, can draw electrons from other chemicals in order to hasten a process.
But metals bring a large collection of issues with them. Many of them are rare and therefore expensive; obtaining them often involves large mining operations. They can also be indiscriminate, catalyzing alternate reactions at appreciable levels or engaging in reactions themselves, which can deactivate their catalytic functions. All of which makes finding alternatives to metal catalysts a valuable pursuit.
And in fact, we know there are alternatives. Some of the most effective catalysts on the planet are enzymes that are made entirely of inexpensive and easy-to-obtain materials like carbon, nitrogen, oxygen, and sulfur. Over the years, various reports have appeared in the literature showing organic chemicals—the ones made of these elements—acting as useful catalysts. The problem is that the results turned out to be isolated. Nobody followed up on them, and they weren't used to build a comprehensive understanding of the principles behind what are now termed organocatalysts.
In 2000, both List and MacMillan published papers that helped change that situation. Rather than focusing on the reactions and catalysts used in these papers, we'll discuss some of the general principles that can be extracted from them, since those ideas are what really drove advances in the field.
Why organocatalysts are potent
As mentioned above, metal catalysts often help move chemical reactions along by donating or receiving electrons. Metals work well here because it frequently takes very little energy to change the number of electrons they have. Adding or removing electrons from something like carbon involves considerably more energy. But organic molecules often distribute their electrons across bonds that are spread across multiple atoms. Temporarily adding or removing electrons from these systems of chemical bonds can require much less energy, allowing them to function a bit like a metal.
This sort of behavior can be enhanced by strategically located nitrogen atoms, which have two additional electrons that can't take part in these bonding networks. In the right circumstances, the atoms can participate in the transfer of electrons as well.
Another feature of organocatalysts is one they share with many enzymes. Enzymes often function by interacting with the molecules in the reaction they catalyze in a way that stretches or strains them. In many cases, the altered geometry of the molecules ends up being similar to the geometry of an intermediate in the chemical reaction. This process ultimately makes the reaction much more likely to occur (which is the job of a catalyst).
And while they're not as large and complex as enzymes, organocatalysts can sometimes perform these functions via hydrogen bonding or hydrophobic interactions between the catalyst and one or more of the molecules engaging in the reactions.
Finally, many catalysts (including metals) form temporary chemical bonds with one of the molecules involved in the reaction. In other words, one of the intermediate chemicals in the reaction is a combination of the catalyst and one of the molecules involved in the reaction. This intermediate chemical then reacts with another molecule, driving things forward.
This process has a significant side effect. Many of the organic chemicals involved in the reactions can come in two forms (called enantiomers) that are mirror images of each other, like left and right hands. Left on their own, most chemical reactions will produce a mix of the left- and right-handed products. But the form of the intermediate can help enforce a handedness on the final product of the reaction. This lets chemists engineer reactions that mostly produce a single enantiomer.
Different catalysts, developed both by the Nobel winners and people who have built on their work, can take advantage of one or more of these features.
Going green
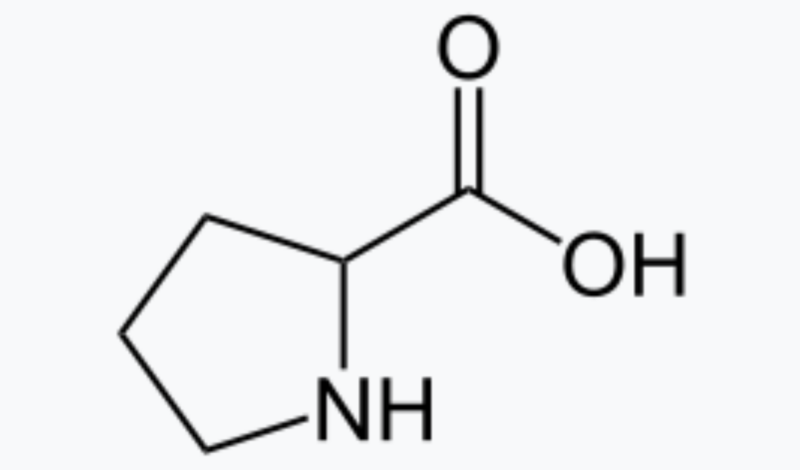
Organocatalysts bring two other big advantages. The first is that the catalysts themselves overlap heavily with biology—one of List's key papers used proline, which is an amino acid that is also incorporated into many protein catalysts. That means chemistry and biochemistry can have a useful two-way conversation, with chemists identifying catalysts that may share mechanisms with enzymes and biochemists suggesting new forms of catalysis in enzymes that might be mimicked by simpler molecules.
Which brings us to the second point. Advancing both enzymes and organocatalysts can make it far, far easier to shift society onto a more sustainable foundation. Any of the elements used to form organocatalysts can be easily extracted from a plant. While we'll still have to mine metals for other reasons, having one less reason could only be a good thing.



3175x175(CURRENT).thumb.jpg.b05acc060982b36f5891ba728e6d953c.jpg)

Recommended Comments
There are no comments to display.
Join the conversation
You can post now and register later. If you have an account, sign in now to post with your account.
Note: Your post will require moderator approval before it will be visible.