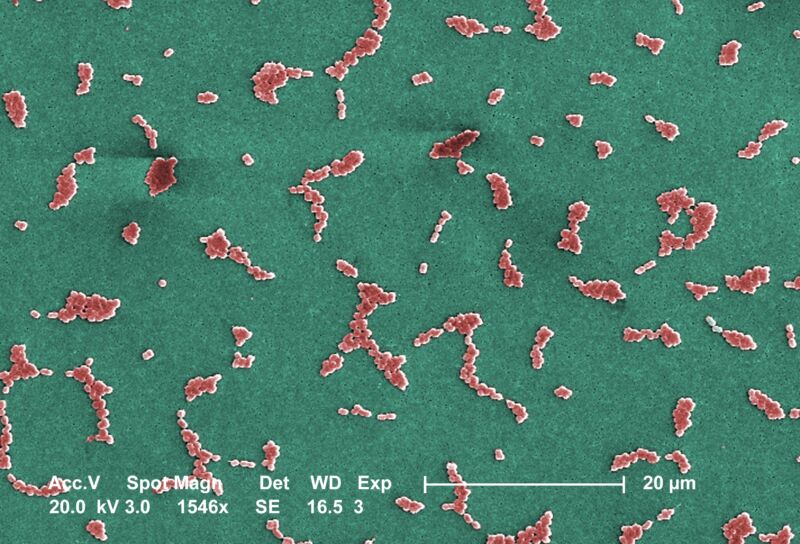
This Scanning Electron Microscope image depicts several clusters of aerobic Gram-negative, non-motile
Acinetobacter baumannii bacteria under a magnification of 24,730x. Members of the genus Acinetobacter are
nonmotile rods, 1-1.5µm in diameter, and 1.5-2.5µm in length, becoming spherical in shape while in their
stationary phase of growth. This bacteria is oxidase-negative and therefore does not utilize oxygen for energy
production. They also occur in pairs under magnification. Acinetobacter spp. are widely distributed in nature,
and are normal flora on the skin. Some members of the genus are important because they are an emerging
cause of hospital acquired pulmonary, i.e., pneumoniae, hemopathic, and wound infections. Because the
organism has developed substantial antimicrobial resistance, treatment of infections attributed to A. baumannii
has become increasingly difficult. The only drug that works on multi-resistant strains of A.baumannii is
colistin which is a very toxic drug.
A new experimental antibiotic can handily knock off one of the world's most notoriously drug-resistant and deadly bacteria —in lab dishes and mice, at least. It does so with a never-before-seen method, cracking open an entirely new class of drugs that could yield more desperately needed new therapies for fighting drug-resistant infections.
The findings appeared this week in a pair of papers published in Nature, which lay out the extensive drug development work conducted by researchers at Harvard University and the Swiss-based pharmaceutical company Roche.
In an accompanying commentary, chemists Morgan Gugger and Paul Hergenrother of the University of Illinois at Urbana-Champaign discussed the findings with optimism, noting that it has been more than 50 years since the Food and Drug Administration has approved a new class of antibiotics against the category of bacteria the drug targets: Gram-negative bacteria. This category—which includes gut pathogens such as E. coli, Salmonella, Shigella, and the bacteria that cause chlamydia, the bubonic plague, gonorrhea, whooping cough, cholera, and typhoid, to name a few—is extraordinarily challenging to kill because it's defined by having a complex membrane structure that blocks most drugs, and it's good at accumulating other drug-resistance strategies
Weighty finding
In this case, the new drug—dubbed zosurabalpin—fights off the Gram-negative bacterium carbapenem-resistant Acinetobacter baumannii, aka CRAB. Though it may sound obscure, it's an opportunistic, invasive bacteria that often strikes hospitalized and critically ill patients, causing deadly infections worldwide. It is extensively drug-resistant, with ongoing emergence of pan-resistant strains around the world—in other words, strains that are resistant to every current antibiotic available. Mortality rates of invasive CRAB infections range from 40 to 60 percent. In 2017, the World Health Organization listed it as a priority 1: critical pathogen, for which new antibiotics are needed most urgently.
Zosurabalpin may just end up being that urgently needed drug, as Gugger and Hergenrother write in their commentary: "Given that zosurabalpin is already being tested in clinical trials, the future looks promising, with the possibility of a new antibiotic class being finally on the horizon for invasive CRAB infections."
An international team of researchers, led by Michael Lobritz and Kenneth Bradley at Roche, first identified a precursor of zosurabalpin through an unusual screen. Most new antibiotics are small molecules—those that have molecular weights of less than 600 daltons. But in this case, researchers searched through a collection of 45,000 bigger, heavier compounds, called tethered macrocyclic peptides (MCPs), which have weights around 800 daltons. The molecules were screened against a collection of Gram-negative strains, including an A. baumannii strain. A group of compounds knocked back the bacteria, and the researchers selected the top one—with the handy handle of RO7036668. The molecule was then optimized and fine-tuned, including charge balancing, to make it more effective, soluble, and safe. This resulted in zosurabalpin.
Deadly drug
In further experiments, zosurabalpin proved effective at killing a collection of 129 clinical CRAB isolates, many of which were difficult-to-treat isolates. The experimental drug was also effective at ridding mice of infections with a pan-resistant A. baumannii isolate, meaning however the drug worked, it could circumvent existing resistance mechanisms.
Next, the researchers worked to figure out how zosurabalpin was killing off these pan-resistant, deadly bacteria. They did this using a standard method of subjecting the bacteria to varying concentrations of the antibiotic to induce spontaneous mutations. For bacteria that developed tolerance to zosurabalpin, the researchers used whole genome sequencing to identify where the mutations were. They found 43 distinct mutations, and most were in genes encoding LPS transport and biosynthesis machinery.
LPS—lipopolysaccharide—is a key bacterial toxin on the outside of Gram-negative bacteria, which are uniquely encased in two menacing layers. There's an outer membrane, which contains LPS, and an inner membrane made of peptidoglycan, a large polymer that forms a rigid mesh scaffold around the bacterial cytoplasm. In between the inner and outer members is a squishy periplasmic space. Any drug that wants to get into a Gram-negative bacterium's cell must traverse both membranes and the periplasm—no small feat. (In contrast, Gram-positive bacteria contain only one thick peptidoglycan layer around their cells. The two types of bacteria are distinguished from each other using a staining method developed by Danish bacteriologist Hans Christian Gram in the 1880s.)
Based on the mutations, it seemed zosurabalpin didn't need to get into the cells to kill the bacteria—it was working in the membranes. Researchers at Harvard, led by Andrew Kruse and Daniel Kahne, worked to figure out exactly what zosurabalpin was up to with LPS-related machinery. The researchers took a multi-pronged approach, using structure, genetic, and biochemical approaches to create a complete picture. First, they solved the structure that revealed what zosurabalpin was up to: It was binding to a complex of lipopolysaccharide transport proteins (Lpt). This transporter complex (aka LptB2FGC) creates a protein bridge that spans from the cytoplasm, through the inner membrane, across the periplasm, and to the outer surface of the outer membrane. Its job is to transport LPS from the inner membrane to its final resting place in the outer membrane. And zosurabalpin was gumming up the works.
Toxic blockage
However, the researchers found that zosurabalpin is not the wrench in the machinery; rather, the drug clamped onto the transporter complex when LPS was in it, in transit. Zosurabalpin trapped the LPS in the transporter, preventing its movement to the outer membrane. This alone isn't lethal for the bacteria—A. baumannii can survive without LPS on its outer membrane. But, while LPS jammed in the transporter en route to the outer membrane, the parts of the machinery at the inner membrane keep working, the researchers found. This creates a toxic buildup of LPS biosynthesis intermediates in the cell. And that is how zosurabalpin kills CRAB.
The LPS transport system has never been the target of an antibiotic before—other classes target things like peptidoglycan, protein synthesis, and DNA replication. The finding opens up a new class of drugs, giving researchers new targets for their drug candidates.
But there are some caveats. For one, zosurabalpin doesn't seem to work on any other Gram-negative bacteria besides A. baumannii. The proteins in the LPS transporter complex are not conserved across different bacteria. Thus, targeting the LPS transporters of other nefarious Gram-negative bacteria will take yet more drug development research. One bright side of this, as Gugger and Hergenrother note in their commentary, is that it may produce species-specific antibiotics, which could protect patients' microbiomes from being obliterated by broad-spectrum drugs, which we now appreciate is bad for human health.
And, of course, with any new antibiotic, there's the inevitability that bacteria will develop resistance. The researchers already found that select mutations in the LPS transporter machinery can knock back the drug's potency. Also, A. baumannii doesn't need LPS to stay alive. That said, simply blocking LPS production would leave A. baumannii more vulnerable, and it's unclear how that trade-off will play out in clinical settings.
For now, zosurabalpin is in early phase clinical trials and researchers are hopeful that it will be the long-sought new antibiotic that's urgently needed.
"With this significant breakthrough, zosurabalpin has the potential to address a major unmet need in the fight against antimicrobial resistance," Kenneth Bradley, lead author on one of the new papers and Global Head of Infectious Disease Discovery at Roche, said in a statement.




3175x175(CURRENT).thumb.jpg.b05acc060982b36f5891ba728e6d953c.jpg)

Recommended Comments
There are no comments to display.
Join the conversation
You can post now and register later. If you have an account, sign in now to post with your account.
Note: Your post will require moderator approval before it will be visible.