
It’s hard to write about battery research around these parts without hearing certain comments echo before they’re even posted: It’ll never see the market. Cold fusion is eternally 20 years away, and new battery technology is eternally five years away.
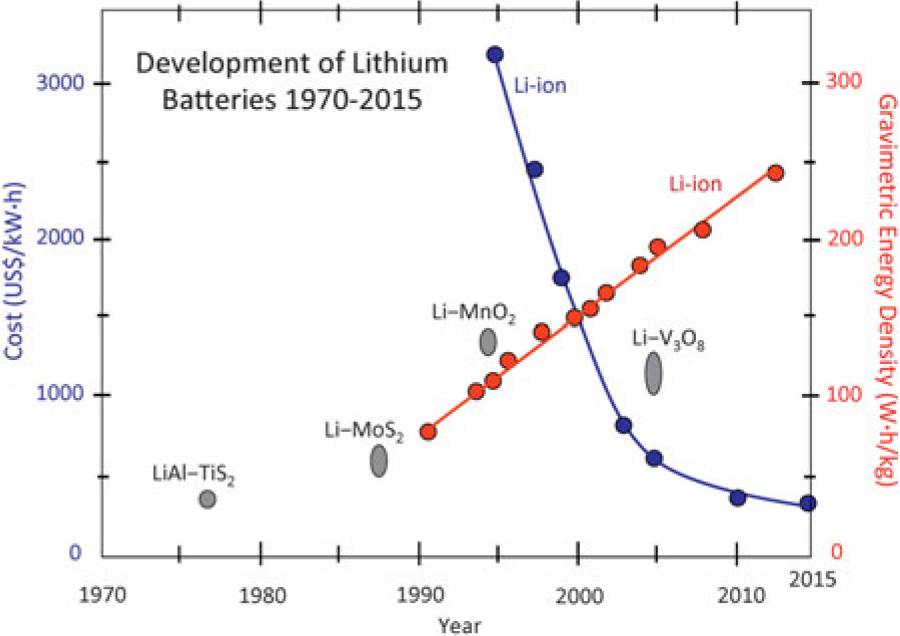
That skepticism is understandable when a new battery design promises a revolution, but it risks missing the fact that batteries have gotten better. Lithium-ion batteries have reigned for a while now—that’s true. But “lithium-ion” is a category of batteries that includes a wide variety of technologies, both in terms of batteries in service today and the ones we've used previously. A lot can be done—and a lot has been done—to make a better lithium-ion battery. In fact, gains in the amount of energy they can store have been on the order of five percent per year. That means that the capacity of your current batteries is over 1.5 times what they would have held a decade ago.
Lithium-ion batteries have evolved, whether you noticed or not. Here's how.
Why does the Li-ion roar?
It’s helpful to start by defining what makes a battery “lithium-ion.” The stars of the show are obviously lithium atoms, which give up an electron easily to form ions. Every battery has a cathode and anode, with a separator and electrolyte sitting between the two. On the cathode side, lithium is found in a metal oxide compound, where it will stay as long as each atom is holding that electron. Once separated from the electron, lithium ions will move across the separator to collect at the anode. The freed electrons can’t cross the separator, so instead they move through whatever circuit is connected to the battery’s two electrodes.
During charging, lithium ions and electrons accumulate in the anode. During discharge, electrons flow through the circuit and lithium ions move through the separator again, reuniting as lithium settles back into the structure of the cathode material.
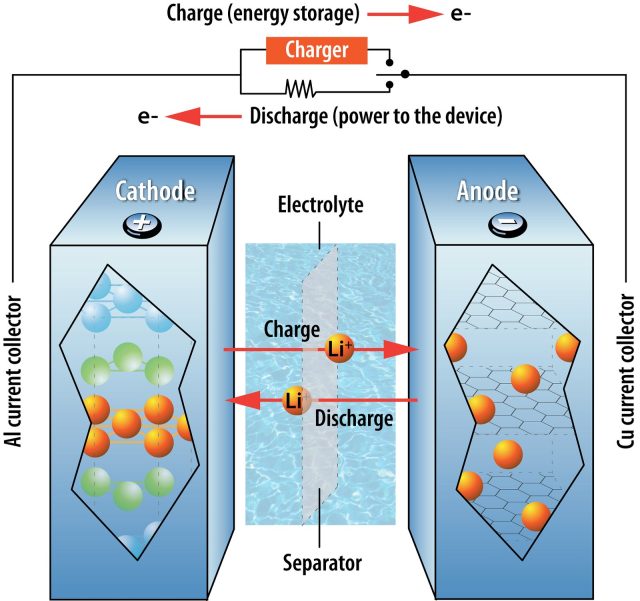
An actual battery is formed from three layers of materials: cathode material deposited on a metal foil, the separator layer, and anode material deposited on another metal foil. Stack these flat, and you have a pouch- or prismatic-style battery like you might find in your phone or a Chevy Bolt. Roll the layers up in a coil, and you have a cylindrical battery like those in power tools or a Tesla.
You can’t get rid of the lithium and still call this a lithium-ion battery, but everything else is fair game. There are many different materials used for the cathode, and you can change the separator or try another chemistry for the electrolyte. There are even options for the anode material, though one has dominated for a long time.
Early attempts at lithium-ion batteries tried using solid lithium metal for the anode, but this produced serious stability problems. (Problems that are still being worked on today.) The breakthrough was the use of graphite for the anode. Graphite consumes valuable space while not contributing additional energy capacity, but its sheet-like structure gives lithium ions safe housing while greatly improving cycle life and safety. This enabled the first Sony lithium-ion batteries in 1991.
Even the first lithium-ion batteries had greater energy density than nickel-metal hydride batteries, holding more charge in less space while weighing less. They also operate with a higher cell voltage, which can be useful. Of course, it’s not all sunshine and unicorns. Lithium-ion batteries are more expensive, and the organic solvent used for the electrolyte is flammable, creating a fire risk that must be carefully managed.
Nickel-metal hydride batteries continue to be used in rechargeable AA and AAA batteries, as well as hybrid vehicles that don’t need as much energy storage. But the lithium-ion battery dominates where space and weight is at a premium, in places like a laptop or electric vehicle.
A very particular set of skills
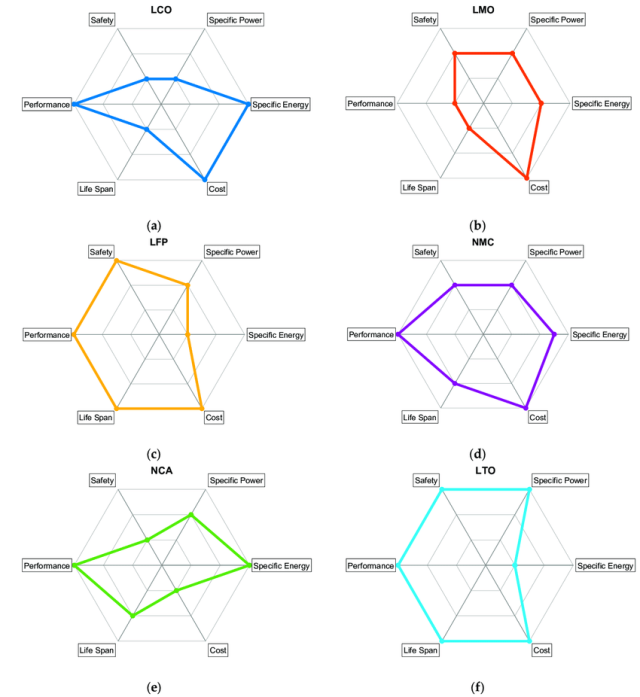
Batteries have more than one or two important characteristics, and so they are often represented by a spider chart (like the one below). “There’s energy density, there is power density, there is cost, there is cycle life, there is calendar life, there is safety,” Argonne National Laboratory’s Venkat Srinivasan told Ars. “What typically happens is that, in batteries, it’s a compromise of these different things.” Even just sticking to lithium-ion batteries, there are configurations and designs that can emphasize certain of these characteristics at the expense of something else. Energy density could be boosted a bit, for example, but maybe it comes at a higher cost or with a reduced cycle life.
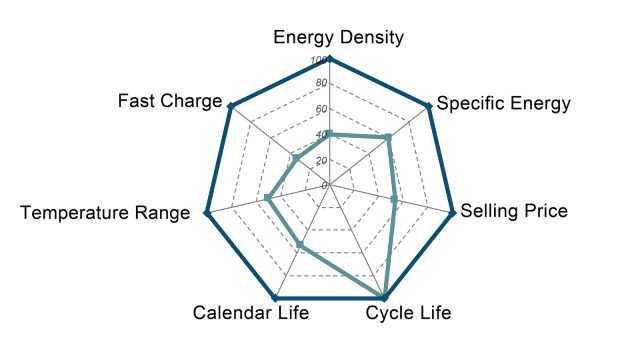
This may be one of the causes of the frustration or skepticism directed toward news about battery research. A study may identify a way to significantly improve one characteristic, generating an exciting top-line conclusion. But the design may be impractically poor in some other way. While battery researchers learn from what does and doesn’t work, this means that a lot of laboratory batteries you may read about will never hit the market.
However, this also means there are a lot of knobs that can be used to customize a specific battery design. Even seemingly subtle things, like the exact thickness of the anode or cathode layer that gets deposited on its metal foil, can affect behavior. The thicker the cathode relative to its foil backing, for example, the greater the energy density of your battery, since less of the total volume is taken up by the foil. But a thicker material layer also means a longer journey for lithium ions and electrons. That generates more heat during battery operation and leads to shorter cycle life. Keep the cathode thinner, on the other hand, and it can handle higher charge and discharge rates, since the shorter journey is easier.
In small devices, where space is at a premium, more expensive designs that maximize energy density are preferred. Electric vehicles are different, since the cost of the battery is a large portion of the overall price—adding a 20 percent premium to the battery could easily push a car beyond your budget. Cycle life has to be much greater, too. Reduced battery life in a phone after two years is generally viewed as par for the course these days. Significantly reduced battery life in a car after two years would be a deal-breaker.
Since electric vehicles are currently on the edge of affordability and (at least for some) acceptable range and charge time, small battery improvements are much more visible here.



3175x175(CURRENT).thumb.jpg.b05acc060982b36f5891ba728e6d953c.jpg)


Recommended Comments
There are no comments to display.
Join the conversation
You can post now and register later. If you have an account, sign in now to post with your account.
Note: Your post will require moderator approval before it will be visible.