Moungi G. Bawendi, Louis E. Brus, and Alexei I. Ekimov laid a vital nanotech foundation.

Vials of quantum dots with gradually stepping emission from violet to deep red.
Antipoff/CC BY-SA 3.0
Once thought impossible to make, quantum dots have become a common component in computer monitors, TV screens, and LED lamps, among other uses. Three of the scientists who pioneered these colourful nanocrystals—Moungi G. Bawendi, Louis E. Brus, and Alexei I. Ekimov—have been awarded the 2023 Nobel Prize in Chemistry by the Royal Swedish Academy of Sciences “for the discovery and synthesisof quantum dots.” The news had already leaked in the Swedish news media—a rare occurrence—when Johan Aqvist, chair of the Academy's Nobel committee for chemistry, made the official announcement, complete with five flasks containing quantum dots of many colours lined up before him as a visual aid.
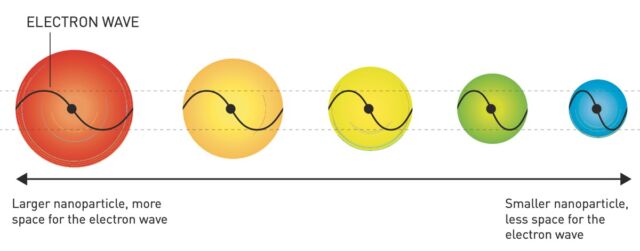
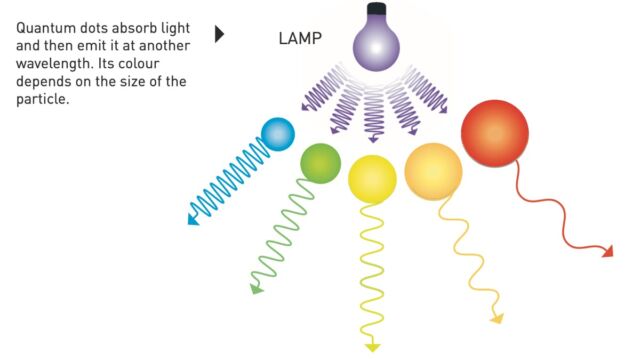
A quantum dotis a small semiconducting bead with a few tens of atoms in diameter. Billions could fit on the head of a pin, and the smaller you can make them, the better. At those small scales, quantum effects kick in and give the dots superior electrical and optical properties. They glow brightly when zapped with light, and the colour of that light is determined by the size of the quantum dots. Bigger dots emit redder light; smaller dots emit bluer light. So, you can tailor quantum dots to specific frequencies of light just by changing their size.
Physicists had thought since the 1930s that particles at the nanoscale would behave differently. That's because, according to quantum mechanics, there is much less space for electrons when particles are that small, squeezing electrons together so tightly that material properties can change dramatically. Scientists succeeded in making nanoscale-thin films on top of bulk materials in the 1970s that had size-dependent optical properties, in keeping with those earlier predictions. But making those films required ultra-high vacuum conditions and temperatures near absolute zero, so nobody expected them to have much practical use.
A solution emerged from the study of ancient coloured glass. Glassmakers long ago realized they could add silver, gold, or cadmium to their molten glass, vary temperature, and control the cooling process to make different shades of coloured glass. Scientists later realized that the colours arose from tiny particles inside the glass, and the particular colour depended on the size of those particles.
In the late 1970s, Ekimov, as a newly minted PhD, began researching the optical properties of coloured glass at the S.I. Vavilov State Optical Institute in what was then the Soviet Union. He drew on some of the optical diagnostic methods he'd used for his doctoral research on semiconductors, shining light on the materials and measuring how it was absorbed to learn more about the crystal structure.
Ekimov began tinting his lab-made glass with copper chloride, X-raying the resulting glass after cooling. He found that tiny crystals of the copper chloride had formed and how he made them—varying the temperature between 500°–700° C and the heating times from one to 96 hours—affected the sizes, which ranged from about 2 nm to 30 nm. Furthermore, the particle size affected the light absorption of the glass, just like the thin films created in the 1970s: the smaller the particles, the more blue light they absorbed. These were the first quantum dots deliberately made in the laboratory.
Alas, Ekimov's 1981 paper announcing his discovery was published in a Soviet journal, and thus, researchers elsewhere in the world didn't have access. That included Brus, who published a 1983 paper announcing his discovery of nanoparticles floating freely in a solution that also showed size-depending optical effects.
Johan Jarnestad/The Royal Swedish Academy of Sciences
While at Bell Laboratories, Brus experimented with nanoparticles of cadmium sulfide, intrigued by their ability to capture light and harness that energy to drive chemical reactions. But he noticed that if he left them on the lab bench for a while, their optical properties would change, suspecting that this occurred because the particles had grown in size. He compared cadmium sulfide particles measuring just 4.5 nm in diameter to those about 12.5 nm in diameter. He, too, found that the smaller nanoparticles absorbed more blue light. The same was true of his subsequent experiments with other kinds of nanoparticles.
A former chief scientist at Nanocrystals Technology in New York, Ekimov was asleep when he got the call from the Academy. “It was really nice that we got it after 40 years of work,” he said. Brus (now a professor emeritus at Columbia University) was also asleep when the phone rang... and rang, and rang. He finally returned one of the calls, which turned out to be from a TV station in Miami keen to get his reaction to the win. "They were the first people to tell me," he said.
Brus said his first reaction was thinking about the many other excellent scientists in the field who had not been awarded a Nobel Prize. "This is a collaborative effort rather than one person making a discovery—partly physics, partly chemistry, partly materials science," he said. "It's a great honor. It's recognition for the field, it's recognition that I worked very hard on this subject for a long time. But at the same time, there are many scientists all over the world who work very hard on their subjects all their lifetime, so I'm just lucky, I guess."
Bawendi was a postdoc in Brus' lab in the late 1980s and joined the intensive effort to address a key shortcoming of his mentor's earlier discovery: The fabrication methods invariably produced nanoparticles of varying and unpredictable quality, particularly about size. This limited the use of quantum dots in practical applications. Brus had improved the process somewhat, but the resulting nanocrystals still weren't quite up to par.
Johan Jarnestad/The Royal Swedish Academy of Sciences
Bawendi figured out the secret to controlling the size of quantum dots in 1993: dynamically varying the temperature during the manufacturing process. First, he injected various substances that can form cadmium selenide into a hot solvent, making sure to saturate the solvent around the needle used for the injections. Small cadmium selenide crystals would immediately form but then stop because the injection cooled the solvent. Then Bawendi would increase the solvent temperature so that the crystals started growing again. The longer he let this process run, the larger the crystals would become, while the solvent ensured a smooth and even surface. Once an easy method for manufacturing near-perfect quantum dots existed, the nanocrystals finally began finding their way into practical applications.
A sleepy Bawendi, now at MIT, initially wasn't sure if the call he received from the Swedish Academy was legit, but soon confirmed that he had been awarded a Nobel Prize. "It's quite an honor," he said. "I’m supposed to teach at 9 am today and I have no idea what’s going to happen." Bawendi also gave high praise to his former mentor, Brus. "I learned so much from him. He molded me as a scientist," said Bawendi. "I try to emulate his style of mentorship with my own students."
“Chemists work continuously to develop and make novel architectures and scaffolds of atoms and molecules designed to deliver tailored properties and function,” American Chemical Society President Judith C. Giordan said in a statement. “Quantum dots are a beautiful example of the ability to theorize a phenomenon, then synthesize and tailor particles and precisely manufacture them. The size tunability of quantum dots allows them to emit specific wavelengths of light for a wide range of uses from displays to bioimaging to lighting applications.”
For instance, quantum dots allow TV manufacturers to precisely tune the emitted colors, producing more accurate hues over a wide range, all while using less electricity. They're useful as an alternative to the organic dyes used to tag reactive agents in fluorescence-based biosensors and have been incorporated into glass windows to essentially turn those windows into photovoltaics, potentially harvesting small amounts of solar energy to offset home energy costs. In 2013, German physicists built the experimental equivalent of Maxwell's demon with a pair of interacting quantum dots. In 2015, scientists made quantum "pee-dots" out of recycled urine and used them to bio-image mouse cells. Future applications might include incorporating quantum dots into flexible electronics, tiny sensors, and solar cells, or using them in encrypted quantum communication systems.
Source




3175x175(CURRENT).thumb.jpg.b05acc060982b36f5891ba728e6d953c.jpg)



Recommended Comments
Join the conversation
You can post now and register later. If you have an account, sign in now to post with your account.
Note: Your post will require moderator approval before it will be visible.