Enzyme mechanisms can be complex, and getting them to work is tricky.
Enzymes are amazing catalysts. These proteins are made of nothing more than a handful of Earth-abundant elements, and they promote a vast array of reactions, convert chemical energy to physical motion, and act with remarkable specificity. In many cases, we have struggled to find non-enzymatic catalysts that can drive some of the same chemical reactions.
Unfortunately, there isn't an enzyme for many reactions we would sorely like to catalyze—things like digesting plastics or incorporating carbon dioxide into more complex molecules. We've had a few successes using directed evolution to create useful variations of existing enzymes, but efforts to broaden the scope of what enzymes can do have been limited.
With the advent of AI-driven protein design, however, we can now potentially design things that are unlike anything found in nature. A new paper today describes a success in making a brand-new enzyme with the potential to digest plastics. But it also shows how even a simple enzyme may have an extremely complex mechanism—and one that's hard to tackle, even with the latest AI tools.
Ending esters
The reaction the research team worked on (involving some of the same people who designed snake venom inhibitors) is the breakdown of what's called an ester bond. Ester bonds are formed by linking two chains of carbon atoms by an oxygen atom, with one of the flanking carbons being linked to a second oxygen. These can be broken apart by adding a water molecule, which leaves one carbon chain linked to an alcohol (COH) group and the other an organic acid (COOH).
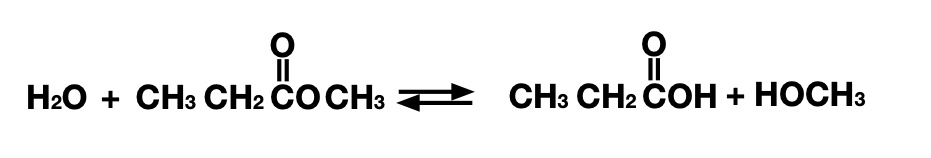
These bonds show up in a variety of biomolecules, so there are many enzymes that can manipulate them. But beyond biology, they also show up in a number of plastic polymers that we use on a large scale—polyester got its name due to how many instances of the chemical bond show up in it. So there's a lot of potential value in being able to break down ester bonds. And we have plenty of examples from biology to show us how it's done.
In this case, though, biology tells us that what looks like a simple chemical reaction can be made incredibly complex. As part of a series of reactions that break the ester into two parts, one of the parts ends up being chemically linked to an amino acid in the enzyme itself. That bond must be broken again by one of the other reactions, or the enzyme becomes inactivated.
To get all these reactions to work, the enzymes all have a key amino acid that's at a critical point relative to the typical pH of living things. That means it can pull a proton from the water surrounding it and donate it to one of the amino acids in the protein. At other points, it rips a proton off an amino acid instead, losing it to one of the parts of the ester. Overall, the simple breaking of one chemical bond has at least four distinct stages and requires multiple amino acids to be positioned within the enzyme's active site with atomic precision.
It's very easy to get an AI tool to design a protein that has the right configuration to do one of these steps. Having it cycle through all four is a different matter entirely.
Need more AI
The researchers started out by using the standard tools they developed to handle protein design, including an AI tool named RFDiffusion, which uses a random seed to generate a variety of protein backgrounds. In this case, the researchers asked RFDiffusion to match the average positions of the amino acids in a family of ester-breaking enzymes. The results were fed to another neural network, which chose the amino acids such that they'd form a pocket that would hold an ester that breaks down into a fluorescent molecule so they could follow the enzyme's activity using its glow.
Of the 129 proteins designed by this software, only two of them resulted in any fluorescence. So the team decided they needed yet another AI. Called PLACER, the software was trained by taking all the known structures of proteins latched on to small molecules and randomizing some of their structure, forcing the AI to learn how to shift things back into a functional state (making it a generative AI). The hope was that PLACER would be trained to capture some of the structural details that allow enzymes to adopt more than one specific configuration over the course of the reaction they were catalyzing.
And it worked. Repeating the same process with an added PLACER screening step boosted the number of enzymes with catalytic activity by over three-fold.
Unfortunately, all of these enzymes stalled after a single reaction. It turns out they were much better at cleaving the ester, but they left one part of it chemically bonded to the enzyme. In other words, the enzymes acted like part of the reaction, not a catalyst. So the researchers started using PLACER to screen for structures that could adopt a key intermediate state of the reaction. This produced a much higher rate of reactive enzymes (18 percent of them cleaved the ester bond), and two—named "super" and "win"—could actually cycle through multiple rounds of reactions. The team had finally made an enzyme.
By adding additional rounds alternating between structure suggestions using RFDiffusion and screening using PLACER, the team saw the frequency of functional enzymes increase and eventually designed one that had an activity similar to some produced by actual living things. They also showed they could use the same process to design an esterase capable of digesting the bonds in PET, a common plastic.
If that sounds like a lot of work, it clearly was—designing enzymes, especially ones where we know of similar enzymes in living things, will remain a serious challenge. But at least much of it can be done on computers rather than requiring someone to order up the DNA that encodes the enzyme, getting bacteria to make it, and screening for activity. And despite the process involving references to known enzymes, the designed ones didn't share a lot of sequences in common with them. That suggests there should be added flexibility if we want to design one that will react with esters that living things have never come across.
I'm curious about what might happen if we design an enzyme that is essential for survival, put it in bacteria, and then allow it to evolve for a while. I suspect life could find ways of improving on even our best designs.
Science, 2024. DOI: 10.1126/science.adu2454 (About DOIs).
Hope you enjoyed this news post.
Thank you for appreciating my time and effort posting news every day for many years.
News posts... 2023: 5,800+ | 2024: 5,700+ | 2025 (till end of January): 487
RIP Matrix | Farewell my friend ![]()
- dabourzannan
-

 1
1


3175x175(CURRENT).thumb.jpg.b05acc060982b36f5891ba728e6d953c.jpg)
Recommended Comments
There are no comments to display.
Join the conversation
You can post now and register later. If you have an account, sign in now to post with your account.
Note: Your post will require moderator approval before it will be visible.